NovoMatrix® Reconstructive Tissue Matrix
The next generation soft tissue augmentation material
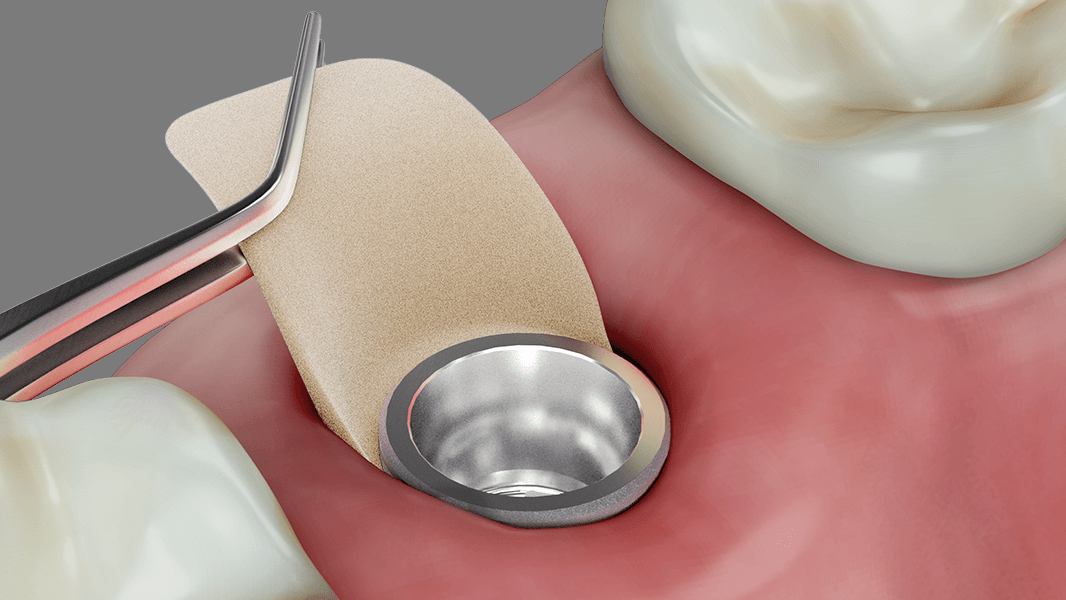
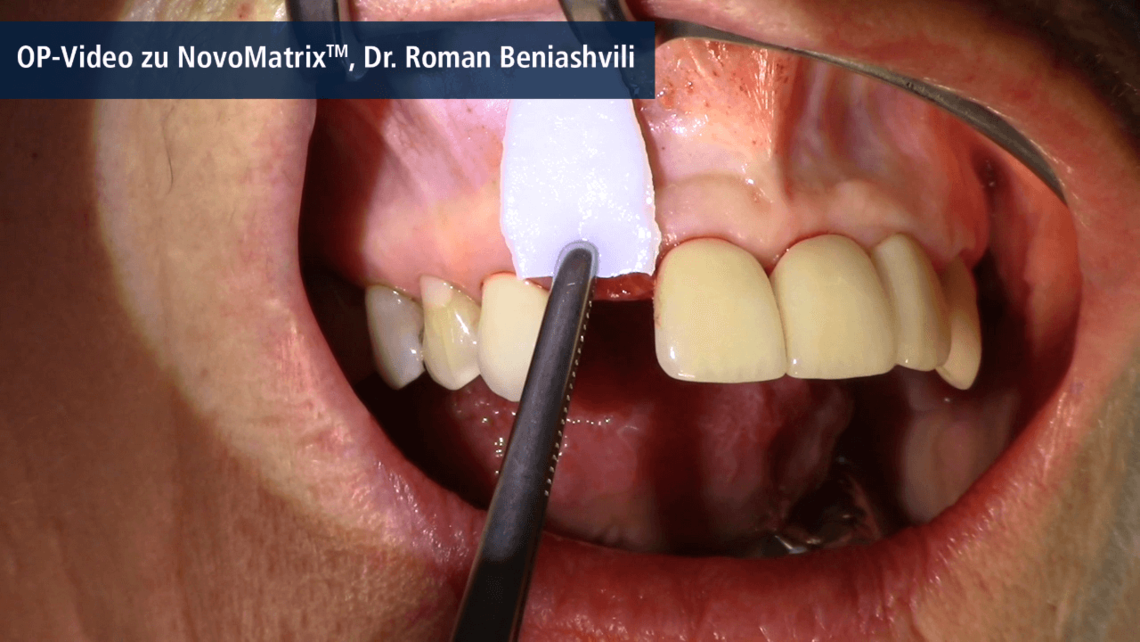
NovoMatrix® is an acellular dermal matrix derived from porcine tissue. In surgical application, the tear-resistant and easy-to-handle1, 2 matrix is an excellent alternative to autologous connective tissue grafts (CTG). There is no need for an intraoral surgical donor site, which reduces morbidity for the patient.
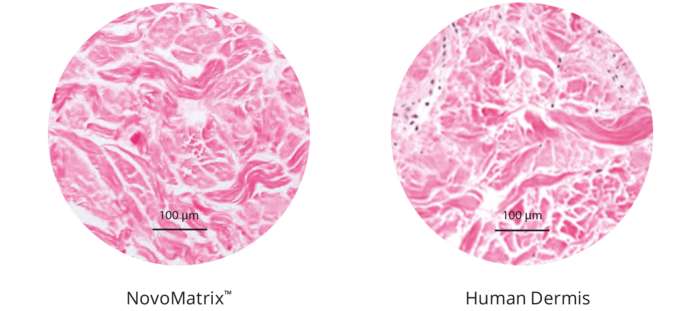
Owing to the manufacturing process, the matrix is free of donor cells. At the same time, the structure of the source tissue remains virtually unchanged, thus supporting the ingrowth of cells and micro-vessels. Proprietary tissue processing enables optimal cell repopulation and revascularization through gentle preparation, resulting in esthetic soft tissue regeneration.3
NovoMatrix® is supplied pre-hydrated in a patented aqueous phosphate-buffered solution containing matrix stabilizers and can therefore be used promptly without requiring extensive rehydration.4
NovoMatrix Optimal for the following indications⁴
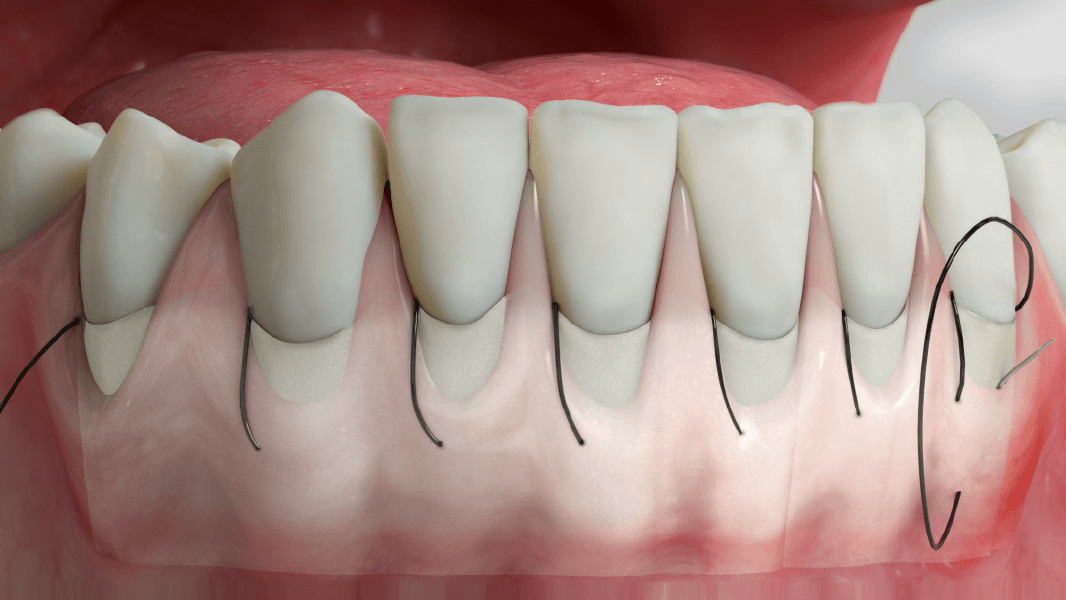
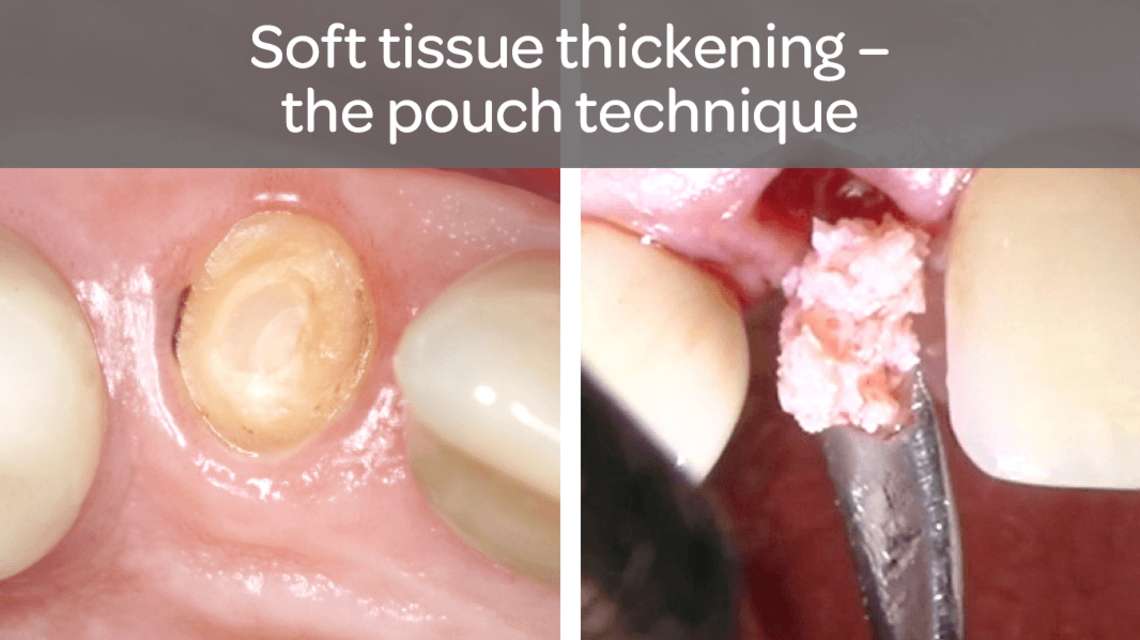
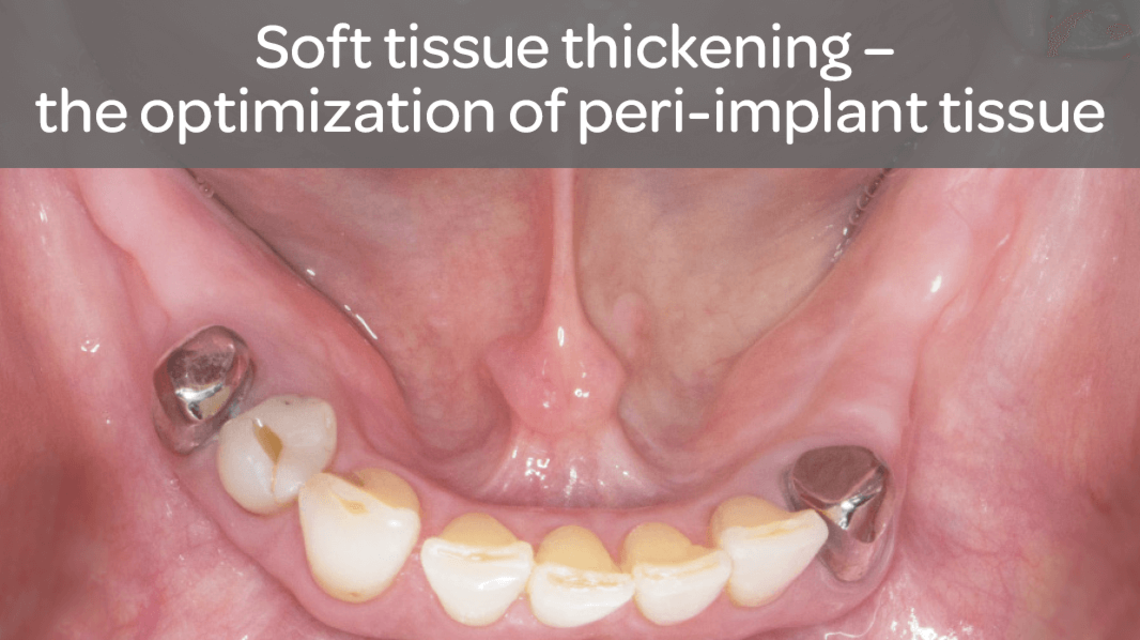
- Increase in attached tissue around teeth and implants
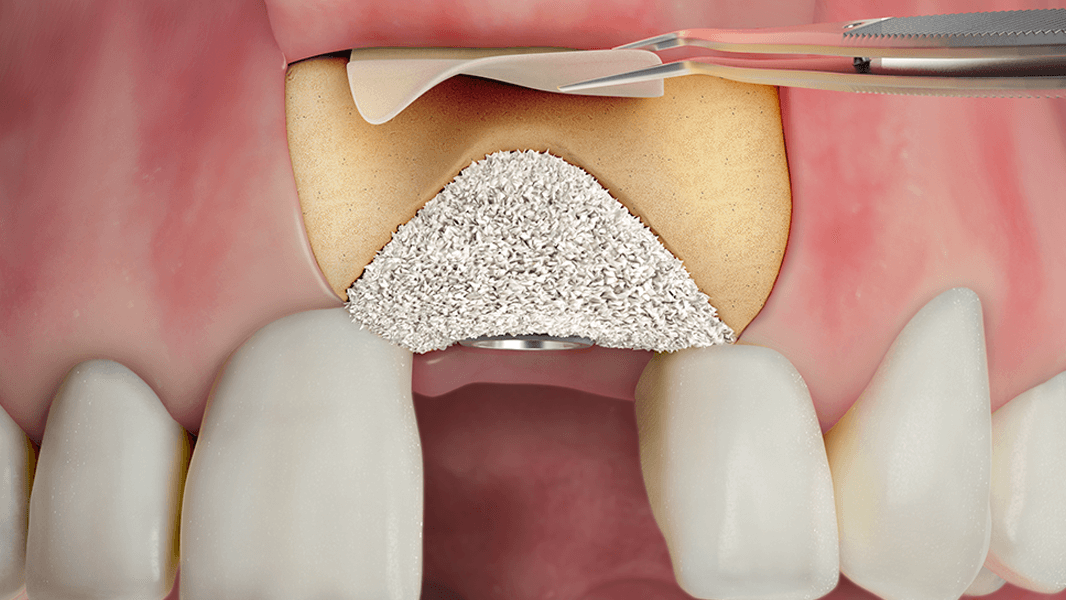
- Reconstruction of the alveolar ridge for prosthetic restoration
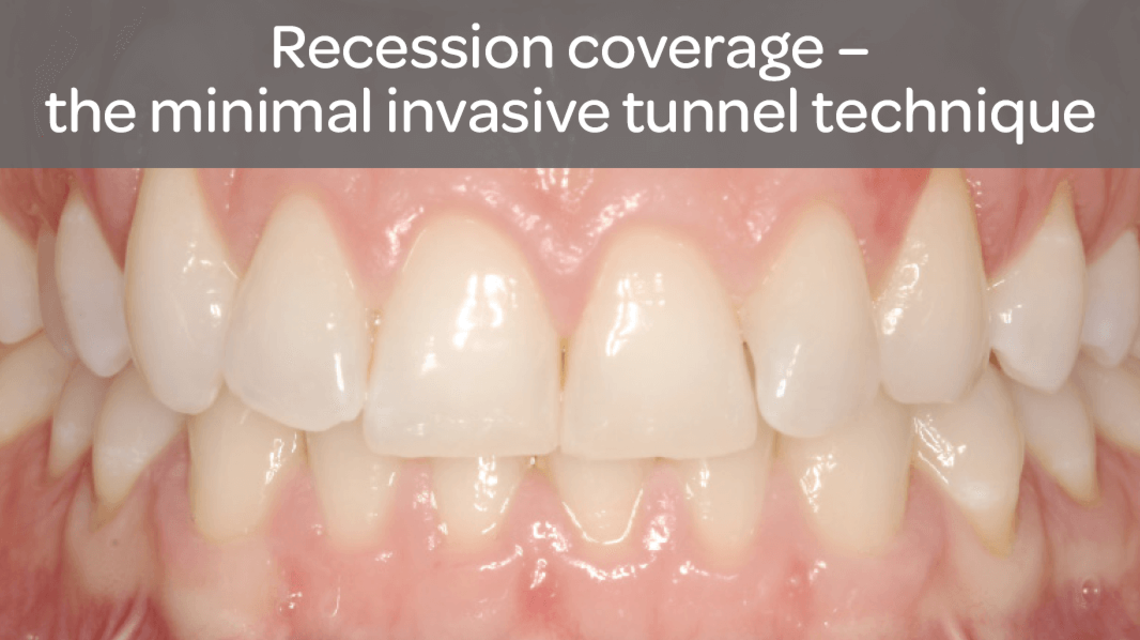
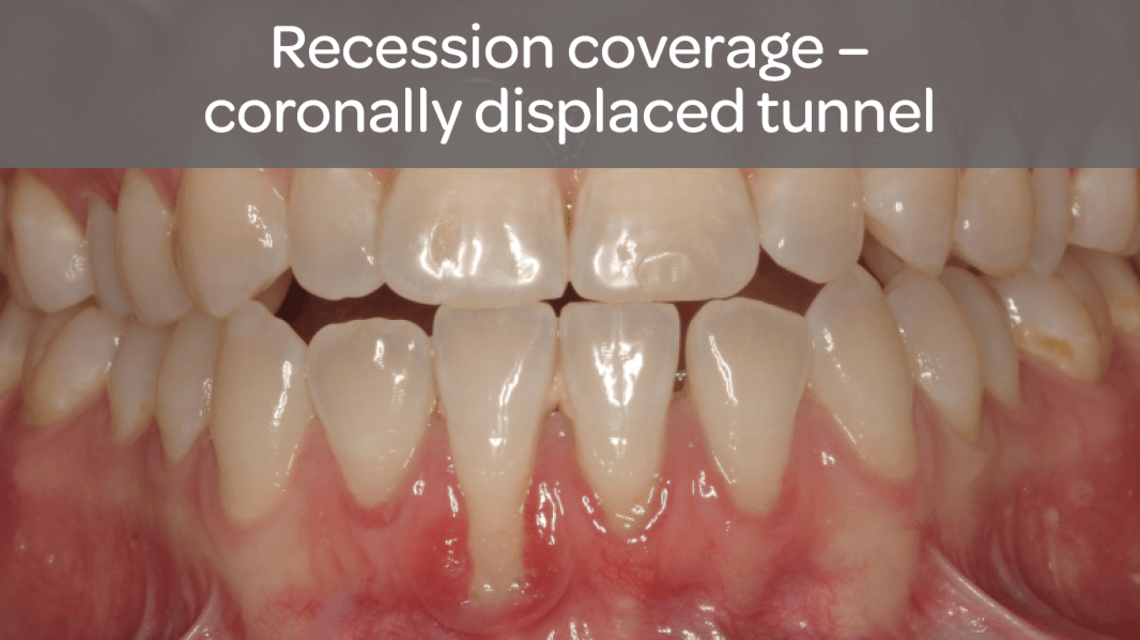
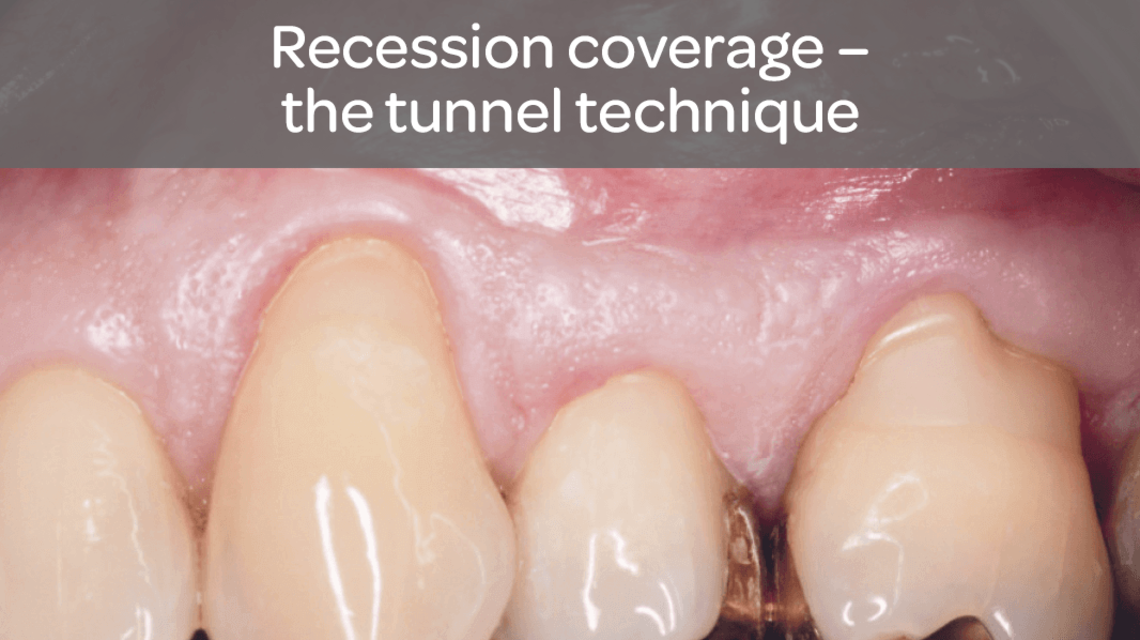
- Guided tissue regeneration in recession defects for root coverage
Product characteristics of NovoMatrix
- The LifeCell™ tissue preparation process results in rapid revascularization.
- Consistent tissue thickness at all times
- Pre-hydrated – ready-to-use out of the package following a 2-minute soak in sterile saline or lactated Ringer’s solution4
- Storage at -8° C to +30° C4
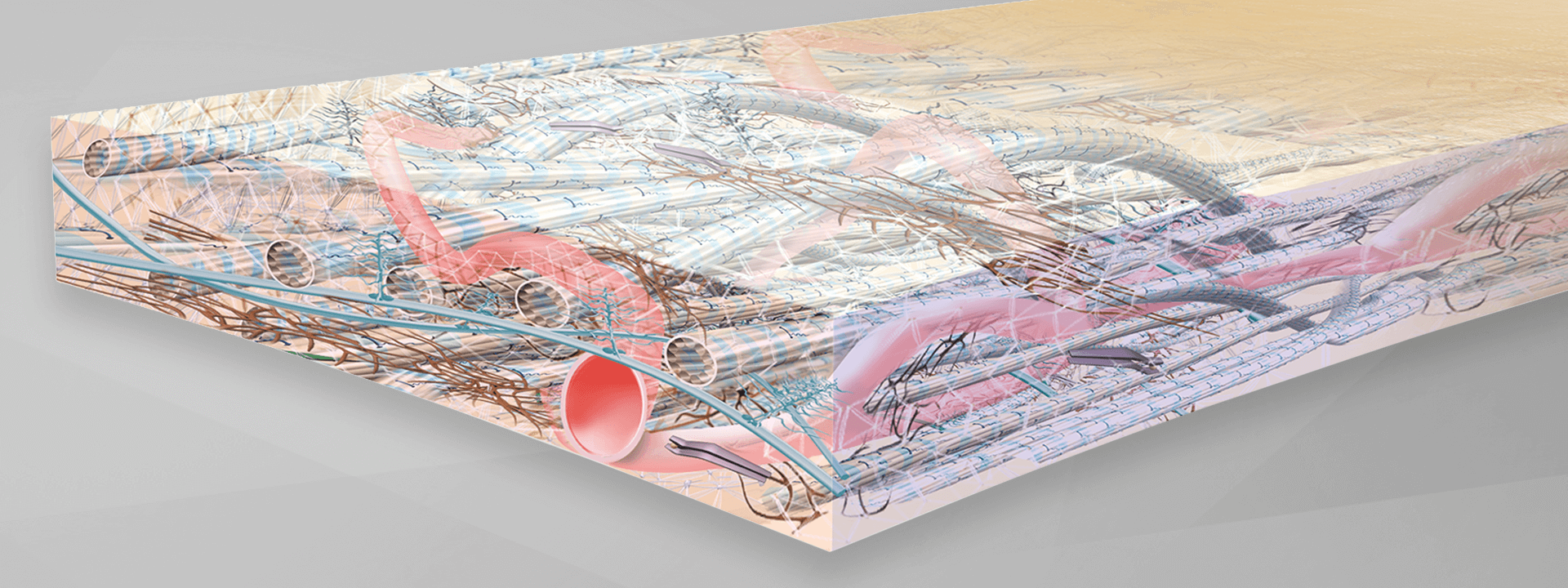
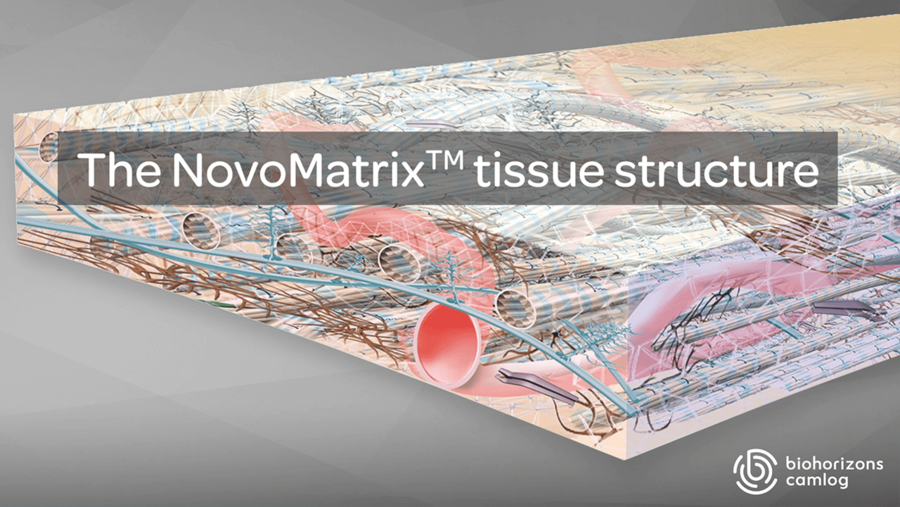
The NovoMatrix tissue structure
Maintenance of graft integrity is essential to achieving successful biological outcomes. NovoMatrix is minimally manipulated and processed gently to ensure it retains components critical to maintaining the biochemical and biomechanical integrity of the tissue.
Advantages of NovoMatrix application
- Shorter surgery time
The ready-to-use collagen matrix shortens surgery time by eliminating the need for a second donor site.9
- Lower patient morbidity
Avoiding a donor site on the palate eliminates the post-operative pain associated with a second procedure.9, 10, 11
- Excellent tissue integration
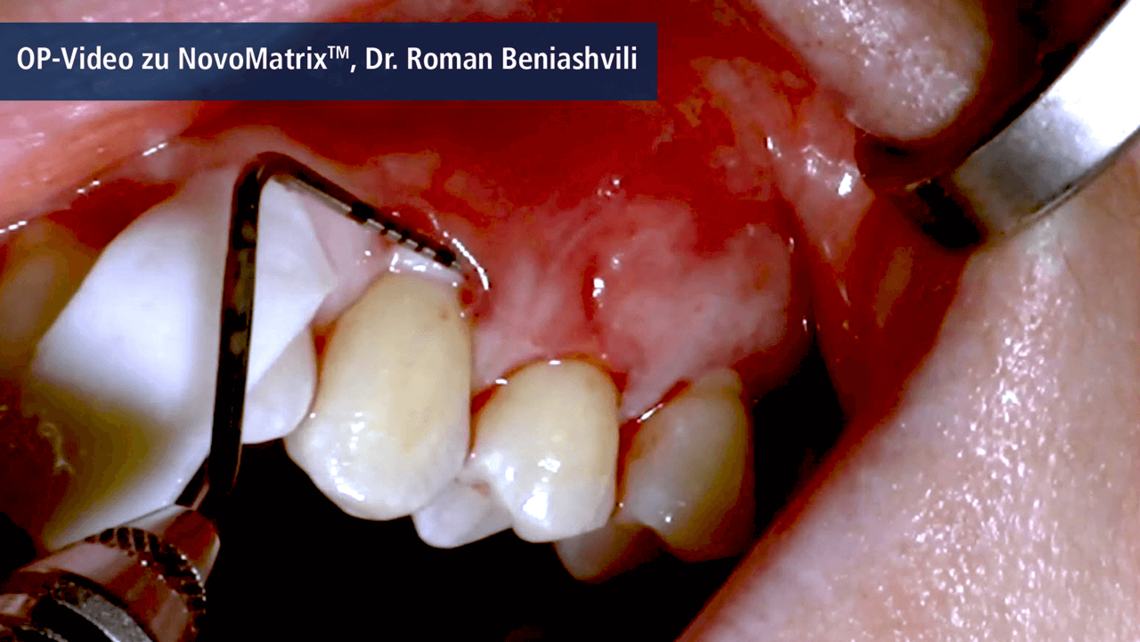
The application of NovoMatrix™ supports rapid revascularization, cellular repopulation and minimal inflammatory reactions.3, 8, 12, 13
- Natural tissue and color structure
The application of NovoMatrix™ demonstrates irritation-free healing and very good adaptation of the color and tissue structure to the natural surrounding tissue.14
- Rapid and complication-free healing of soft tissue
The application of NovoMatrix™ supports a positive immunological reaction as well as tissue integration and regeneration.3, 12, 13, 15
LifeCell™ — over 25 years of experience
Innovative products for tissue reconstruction
For over two decades LifeCell™ has been developing innovative products for a wide range of applications. With over 2.5 million grafts to date, 25 years of experience in tissue processing and an ongoing commitment to innovation, LifeCell™ has joined forces with BioHorizons and Camlog to bring NovoMatrix™, the next generation soft-tissue augmentation material to dentistry.

NovoMatrix instruction manual for use
Please refer to the safety information in the Instructions for Use before using NovoMatrix.
The instructions for use of NovoMatrix can be found in our media center.
Literature
Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices
Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices
Comparison of two soft tissue substitutes for the treatment of gingival recession defects: an animal histological study
References
| 1 | Data on file, Allergan. NovoMatrix™ – Mechanical testing, Preclinical Data. |
| 2 | Data on file, Allergan. INT/0204/2018. |
| 3 | Suárez-López Del Amo F, Rodriguez JC, Asa‘ad F, Wang HL. Comparison of two soft tissue substitutes for the treatment of gingival recession defects: an animal histological study. J Appl Oral Sci., 2019;27:e20180584. |
| 4 | Reference manufacturer’s Instructions for Use (IFU) package insert. |
| 5 | Necas J, Bartosikova L, Brauner P, Kolar J. Hyaluronic acid (hyaluronan): a review. Vet Med. 2008;53(8):397-411. |
| 6 | Ludwig MS. Proteoglycans and pathophysiology. J Appl Physiol. 2007;103:735-736. |
| 7 | Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861-3863. |
| 8 | Harper JR, McQuillan DJ. Extracellular wound matrices: a novel regenerative tissue matrix (RTM) technology for connective tissue reconstruction. Wounds. 2007;19(6):163-168. |
| 9 | Griffin T, Cheung W, Athanasios Z, Damoulis P. Postoperative Complications Following Gingival Augmentation Procedures. J Periodontology 2006;77:2070-2079. |
| 10 | Aguirre-Zorzano LA, García-De La Fuente AM, Estefanía-Fresco R, Marichalar-Mendía X. Complications of harvesting a connective tissue graft from the palate. A retrospective study and description of a new technique. J Clin Exp Dent. 2017;9(12):e1439-45. |
| 11 | Tavelli L, Asa’ad F, Acunzo R, Pagni G, Consonni D, Rasperini G. Minimizing Patient Morbidity Following Palatal Gingival Harvesting: A Randomized Controlled Clinical Study. The International Journal of Periodontics & Restorative Dentistry 38(6):e127-e134 November 2018. |
| 12 | Sandor M, Leamy P, Assan P, et al. Relevant in vitro predictors of human acellular dermal matrix-associated inflammation and capsule formation in a nonhuman primatesubcutaneous tissue expander model. Eplasty. 2017;17:e1-e21. |
| 13 | Xu H, Wan H, Sandor M, et al. Host response to human acellular dermal matrix transplantation in a primate model abdominal wall repair. Tissue Eng Part A. 2008;14(2):2009-2019. |
| 14 | Van Orten A. Peri-implant thickening of soft tissue – stable and functional. Implantologie Journal 5 |
| 15 | Sandor M, Xu H, Connor J, et al. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A.2008;14(12):2021-2031. |
| 16 | Data on file, Allergan. LRD2011-08-015. |
| 17 | Data on file, Allergan. LRD2013-02-004. |